| type |
chains |
composition of triple helix |
triple helix length, structural details |
occurrence, specialities |
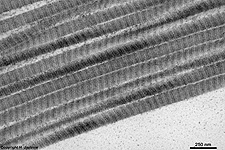
| collagens with long fibrils with 67 nm
periodicity of bands fibrils may consist of different types of collagens,
e.g. type I + III + V or type II + XI |
|
I
|
a1(I); a2(I) |
[a1(I)]2[a2(I)] |
300 nm; diameter of fibrils 50 to maximal 200 nm
chains 1.050 amino acids long |
subcutis, tendon,
bones,
dentin,
Bowman's
membrane, sclera & cornea
of the eye
in nearly all kinds of connective tissues
but not in cartilage |
|
If
|
a1(I); a2(I) |
[a1(I)]3 |
300 nm |
foetal form |
|
II
|
a1(II) |
[a1(II)]3 |
300 nm; diameter of only ~30 nm;
covered by a viscous matrix of proteoglycans |
hyaline- and fibrous
cartilage, vitreal body of the eye,
chorda dorsalis, tectorial
membrane of the inner ear |
|
III
|
a1(III) |
[a1(III)]3 |
300 nm; diameter of ~40 nm;
usually together with type I |
in type I fibrils, form reticular fibres,
loose
connective tissue, corneal
stroma,
subcutis, muscles,
walls of blood vessels |
|
V
|
a1(V); a2(V);
a3(V) |
[a1(V)]3, [a1(V)]2[a2(V)]
und [a1(V)][a2(V)][a3(V)] |
390 nm; N-terminal globular domain
usually together with type I |
in type I fibrils, foetal tissues and membranes; interstitial tissue,
bones,
Bowman's
membrane,
tectorial membrane,
around
smooth muscle cells,
corium,
placenta |
|
XI
|
a1(XI); a2(XI);
a3(XI) |
[a1(XI)][a2(XI)][a3(XI)] |
300 nm; often together with type II, thin fibres |
in type I fibrils, hyaline cartilage,
tectorial
membrane |
| fibril associated collagens with a triple helix which is
disrupted by globular domains |
|
IX
|
a1(IX); a2(IX);
a3(IX) |
[a1(IX)][a2(IX)][a3(IX)] |
200 nm; N-terminal globular domain;
covalently bound to the surface of type II fibrils |
cartilage, vitreous
body,
tectorial membrane,
binds glycosaminoglycans |
|
XII
|
a1(XII) |
[a1(XII)]3 |
large N-terminal globular domain; cross-like
molecule, bound to the surface of collagen type I |
embryonal tendons, subcutis,
corneal
stroma |
|
XIV
|
a1(XIV) |
[a1(XIV)]3 |
large N-terminal globular domain;
cross-like molecule |
fetal subcutis, tendons |
| fibril associated collagens which form string of beat-like
filaments |
|
VI
|
a1(VI); a2(VI);
a3(VI) |
[a1(VI)][a2(VI)][a3(VI)] |
150 nm; N- and C-terminal globular domain; bands with
periodicity of 150 nm, collagen type I associated |
in most interstitial tissues, at the connection of muscles
to tendons,
pericellular matrix of cartilage,
in the walls of blood vessels, in the endomysium |
| collagens that show leaf-like association |
|
IV
|
a1 to 5(IV) |
[a1(IV)]2[a2(V)]
and other forms |
60 nm long triple helix region and 40 nm long
globular domains; formation of tetramers |
all basement membranes, formation
of a two-dimensional interconnected network,
is secreted by cells of endothelium,
epithelium,
glia
cells and fat cells |
|
VIII
|
a1(VIII); a2(VIII) |
? |
regular triangular gutter |
subendothelial tissue, Descemet's
membrane of the cornea of the eye |
|
X
|
a1(X) |
[a1(X)]3 |
150 nm; C-terminal globular domain |
secreted by hypertrophic chondrocytes
thus present in the
epiphyseal plate during
growth of bones |
| collagens which are anchoring fibrils |
|
VII
|
a1(VII) |
[a1(VII)]3 |
450 nm; dimer; globular domain on both ends |
forms the short anchoring fibrils which connect the basal
lamina of epithelia to
the deeper loose connective tissue
whenever a basement membrane is present |
| collagens of which only the DNA was shown |
|
XIII
|
a1(XIII) |
? |
? |
only the c-DNA of this protein was shown in endothelial
cells |
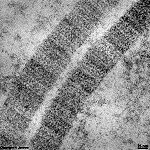
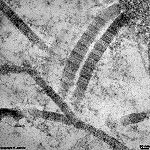
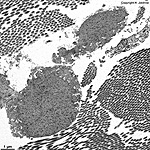
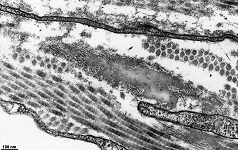
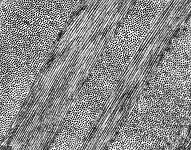
The most common types of collagen are I, II and III. When longitudinally
cut the collagen types I, II, III, V and XI show a characteristic